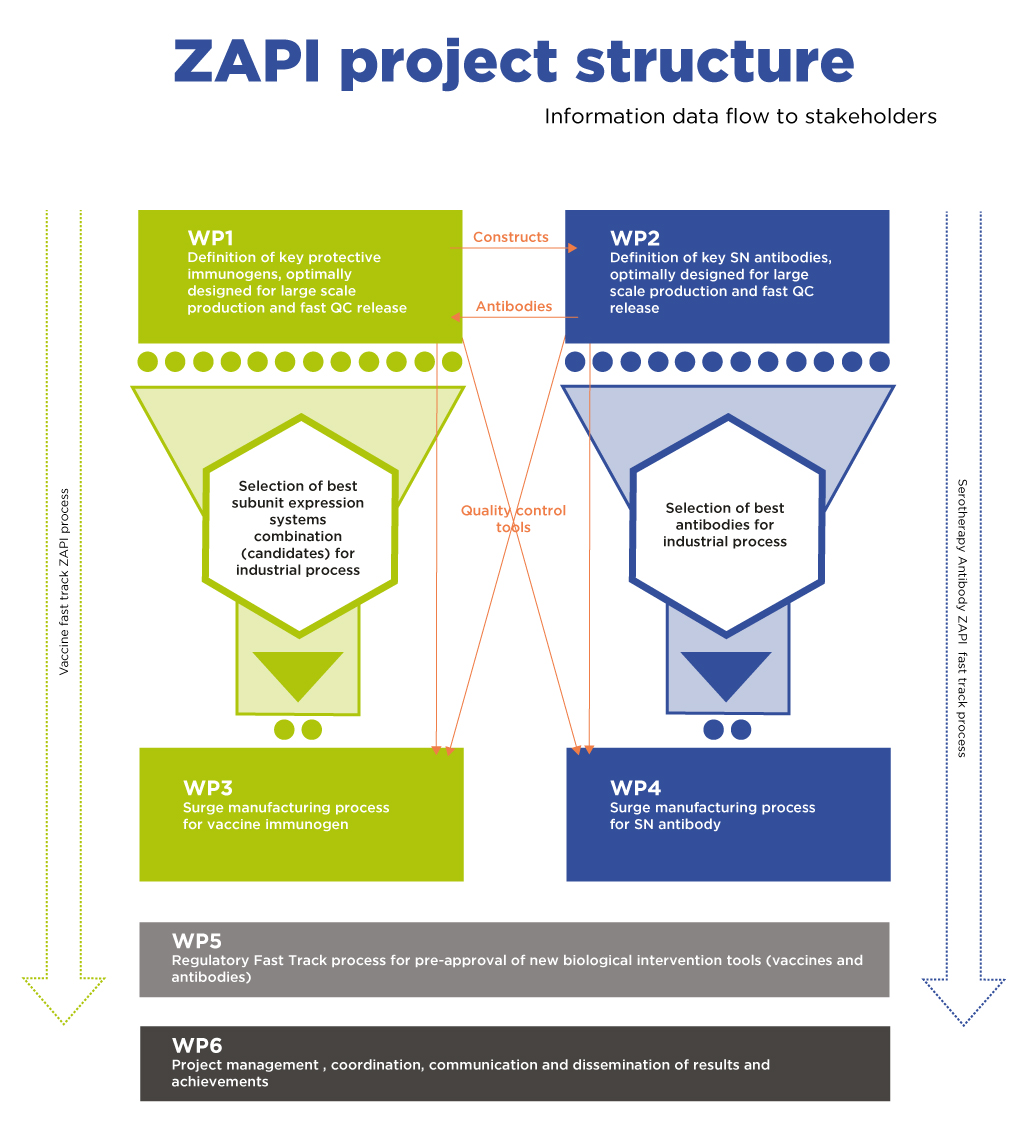
WP1 Innovative antigen-selection and expression pipeline
WP1.1. In silico analysis and antigen prediction (M12)
WP1.2. Expression in non-mammalian expression systems or DNA-launched RNA replicons
In vitro assessment
In vivo assessment in comparison with benchmark when available
Selection of best candidates to enter WP3 for large-scale production (M36)
WP1.3 ZAPI biobase (M0-M60)
WP2 Innovative antibody development pipeline
WP2.1. Humanized antibody and nanobody libraries using WP1 key immunogens (M18)
Development of novel Virus Neutralisation Tests and High-Throughput Screening assays (M24)
WP2.2. Expression in non-mammalian expression systems
In vitro assessment
In vivo assessment
Selection of antibodies to enter WP4 for large-scale production (M36)
WP3 Universal multi-track vaccine expression and production platform
Medium-scale (5-10L) production (M36)
In vivo assessment in small animal and target species models for immunogenicity and efficacy (M48)
Good Manufacturing Practices-like industrial scale (100-250L) production (M48)
In vivo assessment for immunogenicity and efficacy (M60)
Surge vaccine decision tree, driven by industry (M12-M48)
Fast-track in vitro Quality Control procedures and batch release assays (M12-M60)
WP4 Universal neutralizing antibody expression and production platform
Medium-scale (5-10L) production (M36)
In vitro and in vivo assessment (M48 & M60)
Good Manufacturing Practices-like industrial scale (100-250L) production (M48)
Surge neutralizing reagent decision tree, driven by industry (M12-M48)
Fast-track in vitro Quality Control procedures and batch release assays (M12-M60)
WP5 – Regulatory fast-track process for pre-approval of new biological intervention tools (vaccines and antibodies) (M1-M60)
Compliance of protocols with existing regulatory norms of Good Laboratory Practices, Good Manufacturing Practices, Good Clinical Practices, Quality Assurance/Quality Control and identification of potential gaps/obstacles to fast track
Consensus with European Medicine Agency about fast track procedure to be adopted specifically to exploit ZAPI developed processes to respond to pandemic urgencies.
Specific fast track procedure for exploitation in case of urgent need to respond to pandemic urgencies.
Exchange with Public Affairs representatives from Industry and public institutions around the issues raised by the ZAPI project
WP6 Project management, coordination, communication and dissemination of results and achievements (M1-M60)
Scientific and administrative coordination of the project
Maintenance of IMI Grant Agreement and ZAPI Project Agreement
Implementation of the project governance and decision making processes as agreed in IMI Grant Agreement and ZAPI Project Agreement
Impact monitoring
Ethical management
Management of Intellectual Property